
The simulation is produced by Columbia Public Schools, USA. There is also some information on chemical bonds and a cool mystery elements challenge game.
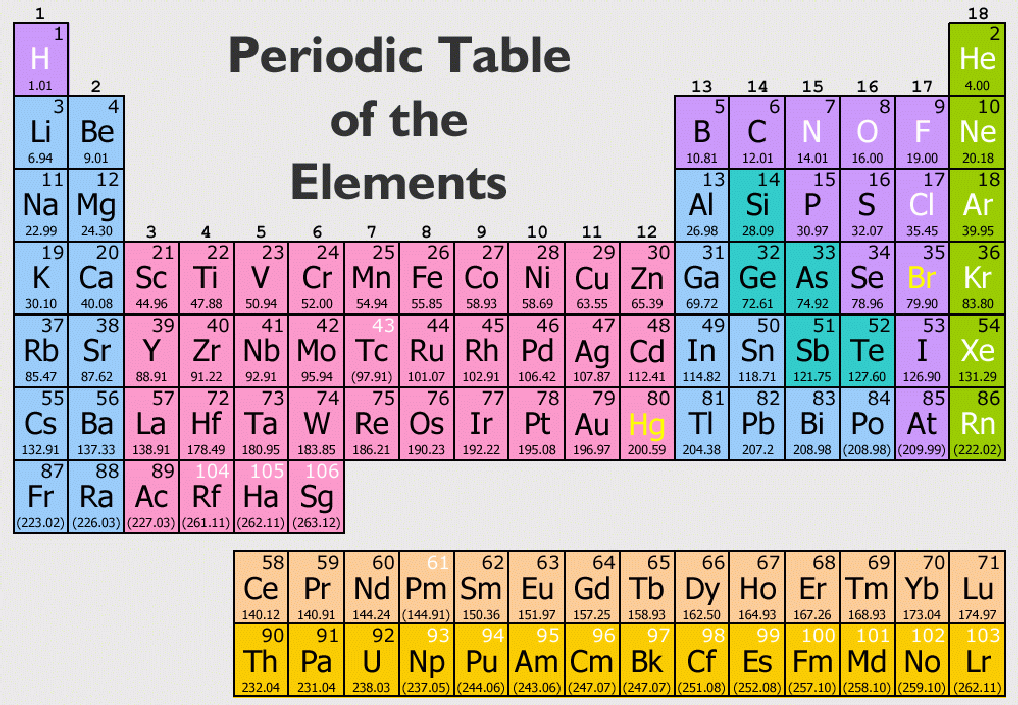
Interactive Periodic Table is an excellent way to explore and learn the Periodic Table. This means their outermost shell of electrons are fully occupied by electrons, which means there is no tendency for the atom to transfer or share electrons during chemical reactions, making it chemically stable. Noble gases have eight electrons in their outermost energy level, except for helium, which has only two electrons. They are unreactive because their atoms have stable arrangements of electrons. Group 0 (or Group 8) elements chemically unreactive The Periodic table starts at element atomic number 1 (this is Hydrogen, symbol H) and ends at element atomic number 118 (Ununoctium, symbol Uuo). Comics include Uncle crooge, Metal Men, Metamorpho, Batman. Metals (like sodium and magnesium) appear on the left side of the Periodic Table, whereas Non-metals (like chlorine, helium and oxygen) appear on the right side of the Periodic Table.Īs you read along a Period (left to right), elements show increasing atomic number. This site contains comic book images linked to the chemical elements via the periodic table. Atoms of different elements have different numbers of protons.Įlements in the same group in the periodic table have the same number of electrons in their highest energy level (outer electrons) and this gives them similar chemical properties. All atoms of a particular element have the same number of protons.Key basic principles about the layout of the Periodic Table: The Periodic Table has 8 Groups: starting from the left is Group 1, then Group 2… Group 8 is also called Group 0. The horizontal rows in the periodic table are called periods and the vertical columns are called groups. This is a table-like chart that shows all the elements with their corresponding symbols- there are just over 100 different elements! Classification, variation, food webs and pyramids.


1.11 Electrode potentials and electrochemical cells (Redox A2).1.10 Equilibrium constant Kc for homogeneous systems (Equilibrium A2).3.6 Organic analysis (AS): analytical techniques.1.7 Oxidation reduction equations (Redox AS).1.6 Chemical equilibria and Le Chatelier’s principle.C3.5 Production of ammonia (an example of a reversible reaction).C3.4 Further analysis and quantitative chemistry.
ATOMIC TABLE FULL


 0 kommentar(er)
0 kommentar(er)
